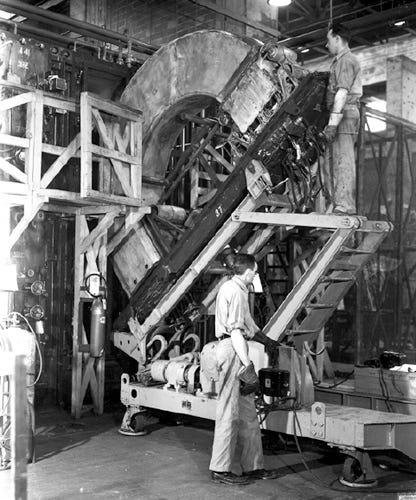
From Treasury Vault to the Manhattan Project
Originally Posted on americanscientist.org
The U. S. Army’s World War II Manhattan Project was a drama unlike any other, with larger-than-life starring personalities, a supporting cast of more than 100,000, cutting-edge science, espionage and diplomatic intrigue. If that wasn’t enough, billions of dollars were gambled in the construction of enormous secret facilities to produce materials for a devastating weapon that might not work.
All this played out against a background of worldwide conflict and the profound threat that Nazi Germany might achieve the world’s most powerful weapon first.
Any compelling drama includes subplots, and the Manhattan Project was no exception. While the main veins of the science, engineering, ethics and geopolitical implications surrounding the development of nuclear weapons have been well mined by historians, some aspects of the Manhattan Project have attracted less study. Over the past few years I have researched several, including the so-called Silver Program. The program was fundamental to finding a means to safely and quickly produce the tens of kilograms of uranium necessary for a weapon of mass destruction.
The secret government project to build the bomb was handed from the Office of Scientific Research and Development to the War Department in 1942. Management fell first to Colonel James C. Marshall of the Syracuse District of the U.S. Army Corps of Engineers. Marshall was ordered to establish a new district with no geographical boundaries. Its first offices were at the Atlantic Division headquarters on Broadway in New York City, hence the origin of the “Manhattan Engineer District” (MED), later called the Manhattan Project. Colonel (soon to be Brigadier General and later Major General) Leslie Groves was appointed commanding general of the new district in September 1942, a position senior to that of Marshall, who remained District Engineer until August 1943 when his deputy, Colonel Kenneth Nichols, replaced him.
The paramount problem for the MED was finding means to produce sufficient enriched uranium for an atomic bomb. By early 1942, only milligram-scale quantities of uranium-235 (235U) had been isolated and it was by no means clear if any of the laboratory methods in use then could be ramped up to an industrial scale. Research into several techniques was fast-tracked and three ultimately were used at Oak Ridge. (A fourth approach to securing fissile material, synthesis of plutonium, was used in giant reactors located in Hanford, Washington.) From the beginning, electromagnetic mass spectroscopy was identified as a promising method, and it quickly became clear to Marshall and Nichols that massive quantities of copper would be needed for the magnets’ windings. But copper—used in shell casings—was a high-priority commodity during the war. So Marshall and Nichols struck on the idea of using silver as a substitute. Congress had authorized the use of up to 86,000 tons of Treasury Department silver for defense purposes. Not having to divert mass amounts of copper was a huge boon for the project’s secrecy.
Nichols met with Undersecretary of the Treasury Daniel Bell on August 3, 1942, to inquire about borrowing 6,000 tons of silver from Treasury vaults. In his memoirs, Nichols relates that Bell indignantly informed him that the Treasury’s unit of measure was the troy ounce—though not all present at that meeting recall any unpleasantness. Many MED documents do report quantities of silver in troy ounces and fine troy ounces. At 480 grains, a troy ounce is somewhat heavier than a common avoirdupois ounce, which weighs in at 437.5 grains. Fine troy ounces (FTOs) refer to the purity of the bullion: a 1,000 troy ounce “silver” bar of fineness 0.900 contains 900 FTOs of pure silver. For convenience, I quote weights in pounds of metal here after accounting for fineness.
It is not uncommon to read Groves described as arrogant, arbitrary, insensitive, overbearing and high-handed. More appropriate labels might be mission focused and supremely competent. Groves graduated fourth in his 1918 West Point class and also trained at the Army Engineer School, the Command and General Staff School and the Army War College. His career in the Corps of Engineers was marked by steady advancement. When he assumed command of the MED, he was deputy chief of construction of the Corps of Engineers and was responsible for all domestic military construction. He had just overseen the building of the Pentagon and was well versed in the capabilities of large-scale contractors. It was Groves who selected J. Robert Oppenheimer to be the scientific director of the Los Alamos Laboratory, where the bomb was designed and built. The biographer Robert Norris described Groves as the project’s “Indispensable Man.” In Nichols’s blunter words: “General Groves is the biggest S.O.B. I have ever worked for. He is most demanding. He is most critical.… He is abrasive and sarcastic.… He is extremely intelligent … abounds with energy … if I had to do my part of the atomic bomb project over again and had the privilege of picking my boss I would pick General Groves.”
One of Groves’s first actions at the MED was to acquire a large tract of relatively isolated land in eastern Tennessee that had been deemed suitable for establishing enrichment facilities. The army evicted some 1,000 families to take possession of a roughly rectangular tract of about 90 square miles, located 20 miles west of Knoxville, to establish the Clinton Engineer Works. The electromagnetic-separation plant, code-named Y-12, was to be built there. Construction of Y-12 was an enormous undertaking, requiring 67 million hours of labor by a workforce that peaked at about 20,000. The complex included more than 200 support buildings and required some 5,000 operating and maintenance personnel. Most had no idea what they were producing until President Truman announced that an atomic bomb had been dropped on Hiroshima.
The need for so much silver at Y-12 emerged from the physics underlying electromagnetic mass spectroscopy. In an optical context, spectroscopy refers to using a prism to separate light into its constituent wavelengths. Similarly, mass spectroscopy separates atoms or molecules by their masses, with a magnetic field playing the role of the prism. The mass spectroscopes used at Oak Ridge were derived from Ernest O. Lawrence’s Nobel Prize–winning cyclotron, which he invented in 1931. Lawrence’s original device had nothing to do with enriching uranium. He invented it in response to a growing crisis in nuclear-physics research. Throughout the 1920s nuclear physicists depended on purely natural phenomena such as alpha decay to supply the “projectiles” used to bombard target elements and induce reactions. But alpha particles (helium nuclei) arising from natural decays are of rather low energy and are readily repelled by the protons residing within the nuclei of even middleweight target elements. For practical purposes, experimenters were restricted to lighter target elements such as aluminum and magnesium. By the late 1920s, they were rapidly running out of potential targets and needed a means to artificially speed up the bombarding projectiles.
Lawrence’s cyclotron opened a vast new experimental horizon for nuclear-reaction researchers. In the device, two D-shaped metal vacuum tanks were placed back to back, with both perpendicular to a strong magnetic field. Ions injected at the center of the tanks were accelerated toward their sides by alternating the tanks’ electrical polarities at high frequency. Meanwhile, the magnetic field, enacting the Lorentz force law, would try to nudge ions into spiral paths. The ions consequently moved in spiral trajectories and would eventually strike the outer walls of the tanks where they reached targets set up to induce the reactions under study. Lawrence’s first cyclotron was about five inches in diameter. By 1939 he had developed one with a 60-inch diameter that required a 220-ton magnet.
In Lawrence’s calutron (a contraction of California University Cyclotron), ionized and accelerated uranium tetrachloride molecules were released into vacuum tanks, which stood vertically between coils of massive electromagnets. Because the force that a magnetic field exerts on a charged particle is perpendicular to the velocity of a particle, the field can do no work on the particle. That is, its speed does not change but its direction does, a textbook application of centripetal force. That is what makes the ions’ trajectories circular.
In this context, researchers discovered that the radius of a given ion’s orbit depends on the strength of the magnetic field, the extent of its ionization, the speed at which it enters the magnetic field and, most importantly, its mass. Ions of greater mass will travel in orbits of larger radii than those of lesser mass. In the case of uranium, two separate ion streams result: one for the rare, fissile isotope of atomic mass 235, which accounts for only 0.7 percent of natural uranium, and one for the common, nonfissile isotope of mass 238. Since the separation of ion streams is widest after only half an orbit, isotopes were collected there. Subsequently, 235U was chemically separated from the tetrachloride molecules.
The design and power requirements of the calutron tanks were simpler than those of the cyclotrons because it was not necessary to generate an electric field inside the tanks. Despite the simplicity of this method on paper, a host of confounding issues arose at Oak Ridge. To sort uranium ions, the magnetic field needed to be very uniform. Molecules ionized differently than what was ideal for the radius of the vacuum tanks splattered against the tanks’ inner walls and had to be scraped out. The resulting collection efficiency was only about 10 percent. Random thermal variations in the ions’ initial velocities inevitably led to some mixing of streams, producing enrichment rather than separation. Complicating things even more, the mass difference between the two uranium isotopes is close to just 1 percent, meaning the stream separation was miniscule unless the magnetic field was extremely strong. And the like-charged ion streams repelled each other and hence displaced the trajectories from ideal curves. This effect limited production rates in individual vacuum tanks to a scant 100 milligrams of 235U per day. To produce the nearly 50-kilogram critical mass of 235U, Y-12 eventually was fitted with more than 1,000 tanks, many containing multiple ion sources.
Lawrence and his Berkeley colleagues developed two basic designs for the Y-12 enrichers: Alpha and Beta units. Alpha units enriched uranium to about 15 percent 235U. That processed material was then fed to the Beta units, which enriched it to bomb-grade level, 90 percent 235U. This meant that the Beta units could be smaller, resulting in savings of power and precious materials. Continuous innovation was a hallmark of the Manhattan Project, and designs for both types of enrichers evolved considerably with experience.
Early in 1943, Groves authorized construction of five Alpha-I enrichers containing 96 tanks with square-shaped coils. The components were arranged in oval configurations called “racetracks.” But the tanks at the curved portions of the oval racetracks were difficult to regulate. In the fall of 1943 the enrichers were supplemented by four Alpha-II tracks, which also contained 96 tanks but were laid out in a rectangular configuration with single units lined up on each side of the racetrack. Beta units contained 36 tanks laid out in a rectangular configuration and used D-shaped coils. Groves initially authorized two Beta units but eventually approved eight, the last of which came on line after the war ended in late 1945. Eventually, nine Alpha and eight Beta units contained a total of 1,152 vacuum tanks.
This is where all that Treasury Department silver comes in. The metal was needed to produce coils to make the calutrons’ giant solenoids, which produced a needed high-intensity magnetic field in accordance with the Biot-Savart law, which describes the strength and orientation of a magnetic field created by an electric current. For the Alpha units, the combination of practicable ion speed and magnetic field yielded ion streams about 3 meters in diameter with a maximum separation of just over a centimeter. Based on a 3-meter side length for the coils and estimating 30 windings for each, one can calculate that the current that flowed through them must have been about 30,000 amperes. At its peak of operations in the summer of 1945, the Clinton Engineer Works consumed about one percent of the electrical power produced in the United States, much of it flowing through those silver coils.
Secretary of War Henry Stimson formally requested the silver in a letter to Secretary of the Treasury Henry Morgenthau Jr. on August 29, 1942. Stimson gave no indication of what the silver would be used for, saying only that the project “is a highly secret matter.” His letter stipulated that the silver should be of fineness 0.999, that title would remain with the United States, and that any silver received by the War Department would be returned in the original quantity, form and fineness to the place from which it was removed. The stated deadline for returning the silver was five years from its receipt or upon written notice from the Treasury that all or any part of it was needed for reasons connected with monetary requirements of the United States. Stimson assured Morgenthau that the metal would be installed only in government-owned plants.
The War Department eventually withdrew more than 400,000 bullion bars of approximately 1,000 FTO each from the West Point Bullion Depository in West Point, New York, a Treasury facility known as “The Fort Knox of Silver.” That amount is equivalent to the weight of about 7,500 midsize automobiles today or some 250 fully loaded World War II B-29 bombers.
The first bars were withdrawn on October 30, 1942, and were trucked about 70 miles south to a U.S. Metals Refining Company facility in Carteret, New Jersey. The next day, the plant began casting the bars into cylindrical billets weighing about 400 pounds each. By the time casting operations ceased in January 1944, just over 75,000 billets weighing nearly 31 million pounds had been cast. Remarkably, this weight exceeded the 29.4 million pounds withdrawn from the Treasury, This was due to very careful cleanup operations of the fabrication facilities. Machines, tools, furnaces, factory floors and storage areas that had accumulated years worth of metal shards were dismantled and scraped clean. Any silver found was separated and cast back into bullion bars. Even workers’ coveralls were vacuumed clean. Armed guards observed every processing step to ensure that all trimmings were recovered. Scrap recovery and cleanup operations were so successful that over the course of processing, more than 1.5 million pounds of silver were collected and returned to Treasury, much of it likely originating from earlier and unrelated silver processing. That more than offset a much smaller amount—11,000 pounds—of borrowed Treasury silver that was unaccounted for.
Once cast, the billets were trucked a few miles north to a Phelps Dodge Copper Products Company plant in Bayway, New Jersey. There the billets were heated and extruded into strips that were 3 inches wide by 5/8 inches thick and 40 to 50 feet long. If all the Manhattan Project silver was shaped today into one strip of that same width and thickness, it would reach from Washington, D. C., to outside Chicago. After being cooled, the strips were cold-rolled to various thicknesses depending on the particular magnet coils for which they were intended. Then they were formed into tight coils (not yet the magnet coils) that were about the size of large automobile tires.
More than 74,000 coils were produced. Most were shipped to Wisconsin for magnet fabrication, but some 268,000 pounds were sent directly to Oak Ridge to be formed on-site into nearly 9,000 busbar pieces. The busbars were massive conductors about a foot square that carried current to the magnet coils. During their manufacture, armed guards again stood by, this time with pieces of paper positioned to catch drill dust as workmen bored holes in pieces of silver in preparation for fastening them together.
The coil strips were shipped from New Jersey to Wisconsin by rail, usually in shipments of six sealed cars, with each shipment containing about 300 coils. The coils were under 24-hour guard—no fewer than three armed guards rode along in a special caboose on each trip. At the Allis-Chalmers Manufacturing Company in Milwaukee, coils were unwound and joined together with silver solder to form larger reels, which were fed into a special machine that wound them around the steel bobbins of the magnet casings. Between February 1943 and August 1944, 940 magnets were wound. On average, each contained about 14 tons of silver. Those coils were then shipped to Oak Ridge on flatcars. These didn’t require guards because the silver was inside welded-shut steel casings.
The pace of work at the Y-12 facility was swift. Ground for the first Alpha building was broken in February 1943, before the facility’s design was even complete and just as the first load of coils was being shipped to Tennessee. The first Alpha track started up just nine months later, on November 13, although its initial operation was short-lived. The windings shorted out because the coils were too close together and because an insulating oil was contaminated with organic material. Operations were halted, and 80 Alpha-I magnets had to be returned to Milwaukee for rebuilding. The second Alpha track entered service on January 22, 1944, and the rebuilt first track on March 3. By late January 1945, nine Alpha racetracks containing 864 calutrons and six Beta racetracks containing 216 calutrons were operating in eight sizable buildings within the Y-12 complex.
The needed isotope accumulated gradually at Oak Ridge. By April 1945, the Y-12 facility had produced only 25 kilograms of bomb-grade uranium and, in conjunction with other enrichment methods, was producing more at about 200 grams per day. By mid-July the facility had produced slightly more than 50 kilograms. By this time Y-12 had consumed about 1.6 billion kilowatt-hours of electricity, about 100 times the energy yielded by the bomb called Little Boy, which was dropped on Hiroshima on August, 6, 1945. Essentially every atom of 235U used in Little Boy was processed in Lawrence’s calutrons. By the end of 1946, Y-12’s cumulative production amounted to just more than 1,000 kilograms of 235U, the equivalent of about 15 Little Boys.
By late 1946, the gaseous-diffusion method of uranium separation was operating much more efficiently than the electromagnetic process, so uranium enrichment in all but one Y-12 building was shut down that December. The last of the Manhattan Project silver, however, wasn’t returned to West Point until June 1, 1970, just a few weeks before Groves died. Some of the Y-12 calutrons continued to be used to separate every element in the periodic table.
After the war, many calutrons were refitted with copper windings. But not the calutrons in what was called the Pilot Plant. They operated until 1974—with 67 tons of silver in their magnet windings until 1970—to separate isotopes other than uranium, some of them used in the graphite reactor in nearby Oak Ridge National Laboratory. That facility created radioactive tracers used in medical tests, an example of wartime technology transformed into a humane use. Other calutrons developed for the Manhattan Project separated stable isotopes until 1998, when cheaper isotope sources forced the operation to close. Sadly, part of the reason that this country faces a shortage of medical isotopes today is because these facilities were shut down.
With the transfer of Manhattan District assets to civilian control under the Atomic Energy Commission on January 1, 1947, Groves saw his influence on nuclear policies wane rapidly, helped in part by the fact that he had offended many influential people over the years. He served as chief of the Armed Forces Special Weapons Project for one year but resigned from the army in February 1948 to become vice president for research at Sperry Rand Corporation, a technology innovator with large contracts with the U.S. military. He held that position until his 1961 retirement. He is buried in Arlington National Cemetery.
Ernest Lawrence continued his advocacy of government-sponsored “big science” projects after the war, including the development of fusion weapons. Frustrated with the slow pace of the fusion program, he teamed with theoretical physicist Edward Teller to lobby for a second weapons laboratory to complement and compete with Los Alamos. In response, Lawrence Livermore National Laboratory was established in California in 1952. In July 1958 President Eisenhower asked Lawrence to travel to Geneva for negotiations with the Soviet Union to develop a treaty banning nuclear weapons testing. The scientist’s long-standing chronic colitis flared up, however, and he was hospitalized. Lawrence died the following month. Element number 103, discovered in 1961 at Lawrence Berkeley National Laboratory, was named Lawrencium in recognition of his many contributions to high energy physics research.
Most of the original buildings in Los Alamos where the bomb was developed were torn down decades ago, but some have been preserved. Los Alamos National Laboratory is still the nation’s primary nuclear-weapons design center, employing more than 10,000 people employed in the stewardship of weapon stockpiles and the manufacture of plutonium cores. The three World War II–era plutonium production reactors at Hanford, Washington were shut down in the 1960s. Two have been sealed up in concrete shells but B-Reactor, the first plutonium production reactor, recently was declared a National Historic Landmark and is being converted into a museum. Environmental remediation at the Hanford site will continue for the foreseeable future.
The Y-12 facility at Oak Ridge still operates as a Department of Energy “National Security Complex” under contract with the Babcock & Wilcox Company. As described in the April 2010 Nuclear Posture Review Report, the Highly Enriched Uranium Materials Facility was recently dedicated there as part of the facility’s mandate to retrieve and store nuclear materials. The ultrasecure warehouse replaces multiple aging buildings with one state-of-the-art storage facility. The report also advocated that a new uranium-processing facility should be built at Y-12 to come online in 2021.
As Richard Rhodes has written, the mammoth scale of the Clinton Engineer Works stands as testimony to the sheer recalcitrance of heavy-metal isotopes. It also testifies to Groves’s desire that this country develop such an unassailable advantage in the production of fission weapons that no country would ever consider a Pearl Harbor–type attack on the United States again. The numerical advantage was secured, though not everyone celebrated the result. Between 1945 and 2009 the United States built more than 66,000 warheads as various designs were developed, tested, deployed and eventually retired, with their fissile material reused in later generation designs. Disarmament treaties have reduced the number of weapons stockpiled in this country in recent decades, although the number of countries worldwide with nuclear weapons has increased.
As this article was written, the National Park Service and the Department of Energy were preparing to submit to Congress a recommendation for a Manhattan Project National Historic Park that will include sites at Los Alamos, Oak Ridge and Hanford. It is my hope that the sites will remain accessible to help future generations explore and understand the project. Historians will forever debate whether it hastened the end of World War II or obviated a bloody invasion of Japan. Some are not convinced that hurrying the end of the war was worth the cost in civilian deaths and the resulting damage to America’s perceived moral standing. But without doubt, the Manhattan Project was the world’s first “big science” undertaking. It affected immediate and long-term events unlike any other science-based endeavor in history. The project and its silver program are exemplars of what sound science and engineering, when conducted by competent practitioners under superb leadership, can accomplish in a time of pressing need. One wonders whether a similar effort could be mounted today.
Gosling, F. G. 2001. The Manhattan Project: Making the Atomic Bomb. Washington: Department of Energy.
Groves, L. R. 1962. Now It Can Be Told: The Story of the Manhattan Project. New York: Harper & Row.
Hewlett, R. G., and O. E. Anderson. 1962. A History of the United States Atomic Energy Commission. Vol. I. The New World, 1939/1946. University Park, PA: The Pennsylvania State University Press.
Jones, V. C. 1985. United States Army in World War II. Special Studies: Manhattan: The Army and the Atomic Bomb. Washington: Center of Military History, United States Army.
Logan, J. 1996. The critical mass. American Scientist 84(3), 263–277.
Nichols, K. D. 1987. The Road to Trinity. New York: William Morrow.
Norris, R. S. 2002. Racing for the Bomb: General Leslie R. Groves, the Manhattan Project’s Indispensable Man. South Royalton, Vermont: Steerforth Press.
Parkins, W. E. 2005. The uranium bomb, the calutron, and the space-charge problem. Physics Today 58(5): 45–51.
Rawlins, B. 1969. Borrowed Silver Worth $1 Billion One of Oak Ridge’s Epoch Secrets. Chattanooga Times, July 14, 1969.
Reed, B. C. 2009. Bullion to B-Fields: The Silver Program of the Manhattan Project. Michigan Academician 39(3): 205–212.
Rhodes, R. 1986. The Making of the Atomic Bomb. New York: Simon and Schuster.
U.S. National Archives and Records Administration, Microfilm set A1218 (Manhattan District History), Reel 10, Book V (“Electromagnetic Project”), Volume 4 (“Silver Program”).
Yergey, A. L., and A. K. Yergey. 1997. Preparative Scale Mass Spectrometry: A Brief History of the Calutron. Journal of the American Society for Mass Spectrometry 8(9): 943-953.